The following post is one of a series previewing the research that will be presented at the held virtually as SciCon (3–7 May 2020, formerly to be held in Dublin, Ireland).
A guest post by Hans Sanderson, Anna Magdalene, Brun Hansen, Scott Belanger, Kristin Connors, and Monica Lam
Polymers are most known for their use in plastics (e.g., polypropylene), and while it is true that all plastics are polymers, it is not true that all polymers are plastics. Polymers have a much wider range of origins and uses and contain a wide variety of materials with differing structural attributes, functionalization, and physical and chemical properties.
They can be both water soluble or insoluble, have synthetic or natural backbones, have different charges and sizes relative to their uses, all of which determine the toxicological and environmental properties of the materials. The different types of polymers are used in a diversity of industries from different composites, over packaging, wastewater flocculants, pharmaceuticals and biocides, personal care products and many other applications. There are thousands of different polymers in use and the global consumption of polymers is increasing. The European Economic Area chemical sales market generated €565 billion profit in 2018 (compared with €542 billion in 2017), with polymers accounting for 21.3% share in total chemical sales in 2018 (20.5% in 2017). For decades, existing polymers have been exempted from regulatory review, although new polymers have required registrations as part of the new chemical registration programs in many countries (e.g., US, China, Canada, Japan, and Korea). Polymers remain an understudied group of chemicals from an environmental toxicity point of view.
A project () is investigating cationic polyquaternium polymers with regards to the assessment of their aquatic hazard. This large group of materials has been receiving renewed regulatory attention after the EU started to review and reconsider its long-standing exemptions of polymers from registrations or detailed risk assessments. This raises a series of questions including: how to assess and model the toxicity of materials that do not comply with more traditional views of toxicity; how to grasp and group materials with little or no publicly available data; how to analyze and verify exposure of highly variable and different materials; and many more. The iTAP Team hosted a addressing the initial layers of this discussion at the 2019 SETAC North America meeting in Toronto and will be further elucidating the topic with a session during the virtual SETAC Europe meeting (SciCon) on 7 May 2020.
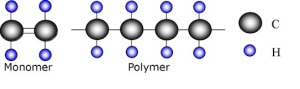
General illustration of polymer structure
Polymers are composed of repeating monomer units. Copolymers are made up of more than one species of monomer. The REACH definition of a polymer is a substance consisting of molecules characterized by the sequence of one or more types of monomer units distributed over a range of molecular weights, wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units. A polymer comprises the following: (a) a simple weight majority of molecules containing at least three monomer units, which are covalently bound to at least one other monomer unit or other reactant; (b) less than a simple weight majority of molecules of the same molecular weight. In the context of this definition a ‘monomer unit’ means the reacted form of a monomer substance in a polymer (). Adding to this complexity is the varying definitions of “polymer” used by regulators elsewhere across the globe, and thus, varying levels of regulatory assessment, scrutiny, and registrations.
Polymers are assumed to be too large to pass through biological membranes and will hence not comply with a classical Paracelsus interpretation of toxicity that “dose makes the poison,” where there is a gradual dose-response induced by intracellular disruptions specific to the chemical. In this light, polymers are of low toxicological concern, given the large size (dimension and molecular weight) of the materials and typical hydrophobicity or receptor driven toxicity is not expected in the environment (). The regulatory exemption of polymers is, however, currently being reconsidered in different regulatory programs, such as EU REACH, as potential concerns are emerging.
Major areas for which challenges remain are touched on below – needs with respect to analytical methodologies, toxicity testing and environmental fate challenges, and challenges encompassing policy development. Analytical challenges to be solved in standardizing characterization for identification and potential grouping, and also for quantification in exposure solutions from ecotoxicity testing, remain to be solved so that conventional labs can analyze and quantify the different materials accurately and precisely at environmentally relevant concentrations.
Considerable challenges also exist when it comes to ecotoxicity. One key driver for such challenges is that the toxicity of polymers is generally limited because their high molecular weight and large steric size limit their ability to cross biological membranes. This makes toxicity testing problematic as some high molecular weight polymers can still exert demonstrable environmental effects. Some polymers are expected to interact with the outer membranes of aquatic organisms and thereby affect their functionalities. This behavior makes it hard to describe the dose-response relationship as responses likely confound external physical effects and internal uptake. As an example, cationic polymers combine two contrasting elements: a positive charge and a long hydrophobic chain, and the resulting dichotomy typically provides unusual behaviors in water, particularly in the interfacial regions. They have low water solubility due to hydrophobicity of the polymer chains, but polymer charge can also modulate toxicity. These mechanical and physical effects are real, and work is being done to understand what descriptors drive these effects for cationic polymers.
Stakeholders are facing challenges in the policy development. What should be the data requirement for cationic polymers in the view of the complex nature of their assessment? How does the legacy of the regulatory priority for polymers affect policymakers’ decisions today? Grouping strategies, exemption and inclusion criteria, and priority setting of chemical entities to be assessed (so-called Polymers Requiring Registration, PRR) calls for new discussions under timings yet to be agreed upon. To do so will also require opening the existing REACH regulation to expand its scope to polymers. Unprecedented levels of private sector-public sector collaboration will be a necessity to be successful in the face of both technical and societal challenges.
During the upcoming virtual SETAC Europe annual meeting (), these challenges will be addressed and discussed in a platform and poster session as a measure to advance the science and inform the new regulatory developments pertaining to polymers.
: 4.10 – Environmental Risk Assessment of Polymers
Presentations will be available on-demand
Session discussion will be held Tuesday, 5 May 2020 | 15.00 – 15.45 UTC | Room 4
