A guest post by Heiko Schoenfuss
The following post is one of a series previewing the research that will be presented at the in Rome, Italy (13-17 May 2018).

Fish A is a normal male fathead minnow. Fish C is a normal female fathead minnow. Fish B is a male that was exposed to female hormones in prescription drugs and looks more like a female than a male. ()
Endocrine disruptors include anthropogenic and naturally occurring chemicals that may disrupt the normal function of the endocrine system. These compounds have reached a near ubiquitous presence in aquatic ecosystems, are capable of interfering with the endocrine system of exposed organisms, and have been linked to a variety of environmental and human health concerns in Europe and elsewhere.
Across the globe, scientists and regulatory agencies have struggled for the past 20 years to evaluate and regulate these compounds, which may occur at low nanogram/liter concentrations (“parts per billion – ppb”) and elicit mostly subtle, sub-lethal effects in exposed organisms. This has led to the development of computer models (in silico), petri dish or culture (in vitro), and live-animal (in vivo) techniques focused on detecting potential endocrine activity, and in vivo tests that collect data that are of immediate environmental relevance (for example, changes in fertility or survival) to detect possible adverse effects.
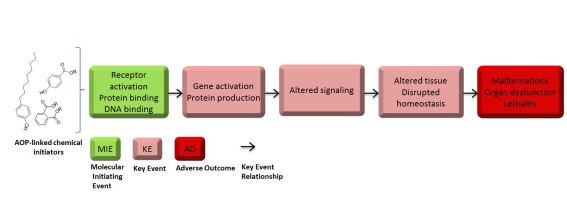
Schematic representation of AOP with hypothetical events
Licensed under CC BY-SA 3.0
A session endorsed by the Endocrine Disrupter Testing and Risk Assessment Interest Group on this topic to be held on Thursday, May 17 at the SETAC Europe annual meeting in Rome will present new methods to address limitations in the assessment of endocrine disruption effects, both in humans and wildlife, incorporating the Adverse Outcome Pathway (AOP) concept in informing the knowledge gaps that are being highlighted in the presentations. The limitations in assessing the effects of endocrine disrupting compounds are related to the characteristics of the endocrine system. Effects of endocrine disruptors may be masked by compensating feed-back loops in the endocrine system, or the expression of effects may be delayed as the endocrine system regulates many long-term biological processes such as development and maturation. Lacking clear dose-response relationships and acute exposure effects creates procedural difficulties in assessing the risk of endocrine disruptors. The oral presentations will examine how to overcome some of these limitations by identifying endocrine disrupting properties in compounds and by using AOPs). AOPs are an exciting recent concept in toxicology that attempts to link effects caused by the interaction between an endocrine disruptor and a molecular receptor in a cell to a cascade of changes in the organism that ultimately creates a linkage to an adverse effect to the organism or its population. These topics will be picked up and expanded on in over 30 poster presentations associated with this session.
Session: Advances in evaluating and regulating endocrine disruptors
17 May 2018 | 10:50 a.m.–12:25 a.m. | Room P
